We are interested in the fundamental mechanisms underlying the functions of cell interfaces. Eukaryotic cells synthesize over 1000 lipid and glycan species and 30% of their genes encode membrane-associated proteins. Together these molecules collectively self-organize into membrane structures that provide important cellular functions. Our vision is to understand and control the connection between the super-molecular organization of cell interfaces and its functions such as cell adhesion, cell mechanics and cell polarity.
Our team develops a combination of fluorescence microscopy, organotypic tissue culture, genetics, and biochemical reconstitution to answer the following questions:
How do adhesion junctions form and function at cell-cell interfaces?
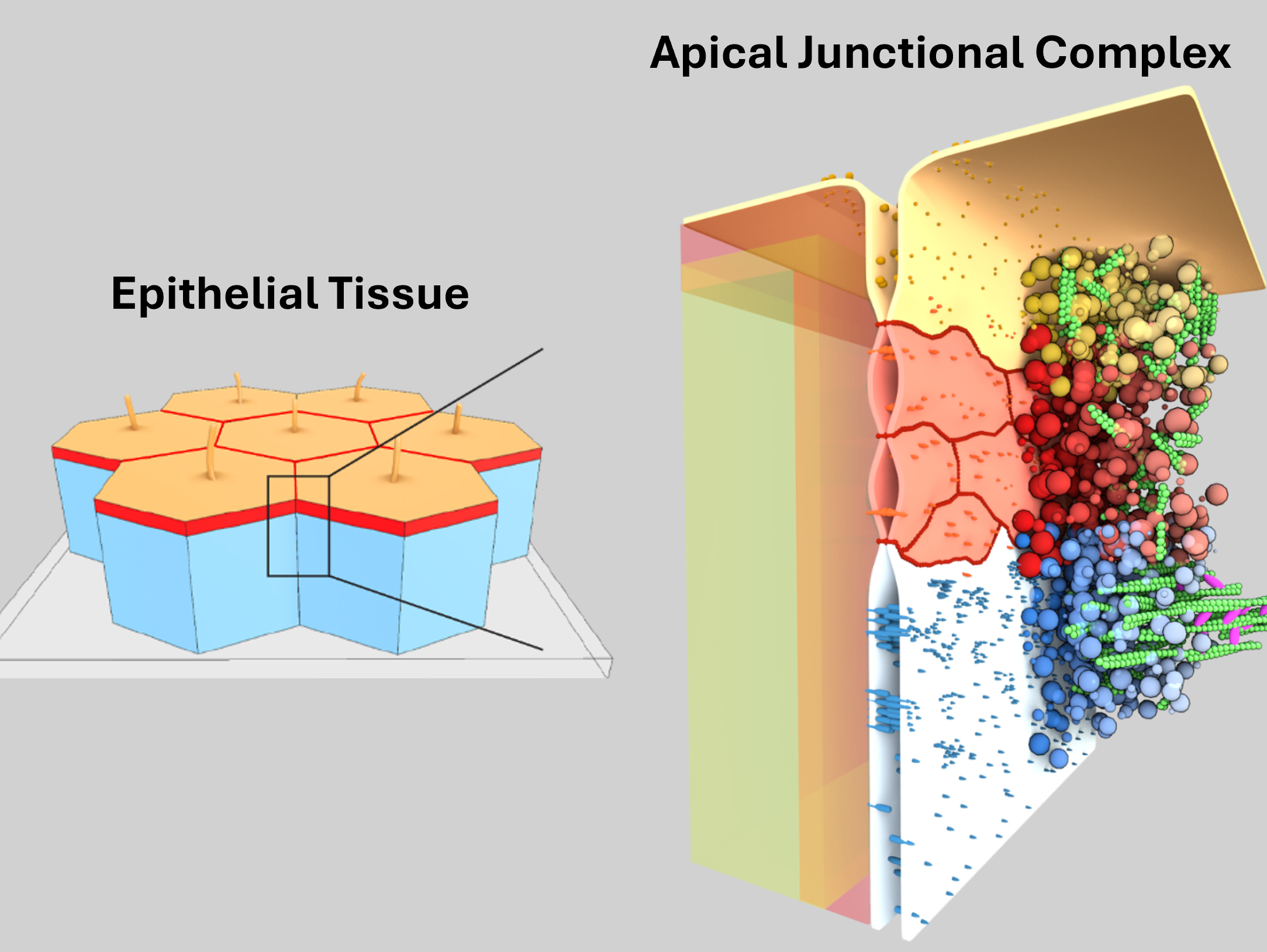
A key structure of epithelial cells is the apical junctional complex, which controls transepithelial solute transport, cell polarity, and tissue mechanics. Using a combination of molecular reconstitution, genetics, and live imaging in tissue culture, we discovered how membrane scaffold proteins control the assembly and shape of the apical junctional complex via membrane-associated phase separation processes (Beutel 2019, Pombo-Garcia 2024, Sun 2025).
How do cells control the lipid composition of their membranes?
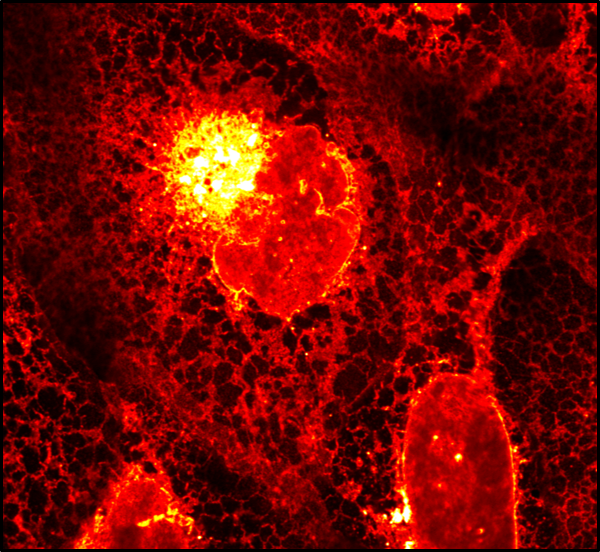
How lipid species are selectively sorted between organelles to maintain specific membrane identities is largely unknown due to the difficulty of imaging lipid transport in cells. Together with the Nadler lab (MPI-CBG), we developed a quantitative lipid imaging pipeline, which allows us to directly visualize transport and metabolism of individual lipid species in mammalian cells using time-resolved fluorescence imaging of bifunctional lipid probes in combination with ultra-high resolution mass spectrometry and mathematical modeling (Iglesias 2025). Our results provide the first quantitative map of lipid flux in cells. We anticipate that quantitative mapping of lipid flux through physical and chemical space in cells will boost our understanding of lipids in cell biology and disease.
How do cells promote the formation of tissue cavities?
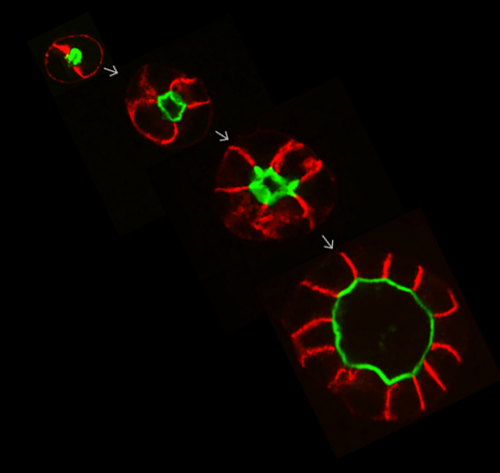
Formation of fluid-filled cavities by epithelial tissues is essential for organ development. How cells control the hydraulic and cortical forces to control lumen morphology is not well understood. We quantified the mechanical role of tight junctions in lumen formation using MDCK-II cysts. We found that the paracellular ion barrier is not required for the hydraulic inflation of a lumen. Instead, tight junctions promote lumen inflation by decreasing cortical tension via the inhibition of myosin Mukenhirn 2024.